Jiapei Hu, Chao Wu, Junqi Weng, Yihui Wang, Xianghong He. Influence of Ferrous Metal Chloride on Photoluminescence Properties of Fully-Inorganic Lead Halide Perovskite Nanocrystals[J]. Laser & Optoelectronics Progress, 2019, 56(10): 101602
Search by keywords or author
- Laser & Optoelectronics Progress
- Vol. 56, Issue 10, 101602 (2019)

Fig. 1. XRD patterns of samples. (a) CsPbBr3 and as-obtained samples prepared by ferrous metal chlorides as chlorine sources (from top to bottom, NiCl2, CoCl2, FeCl2, and FeCl3 used as chlorine sources); (b) local patterns
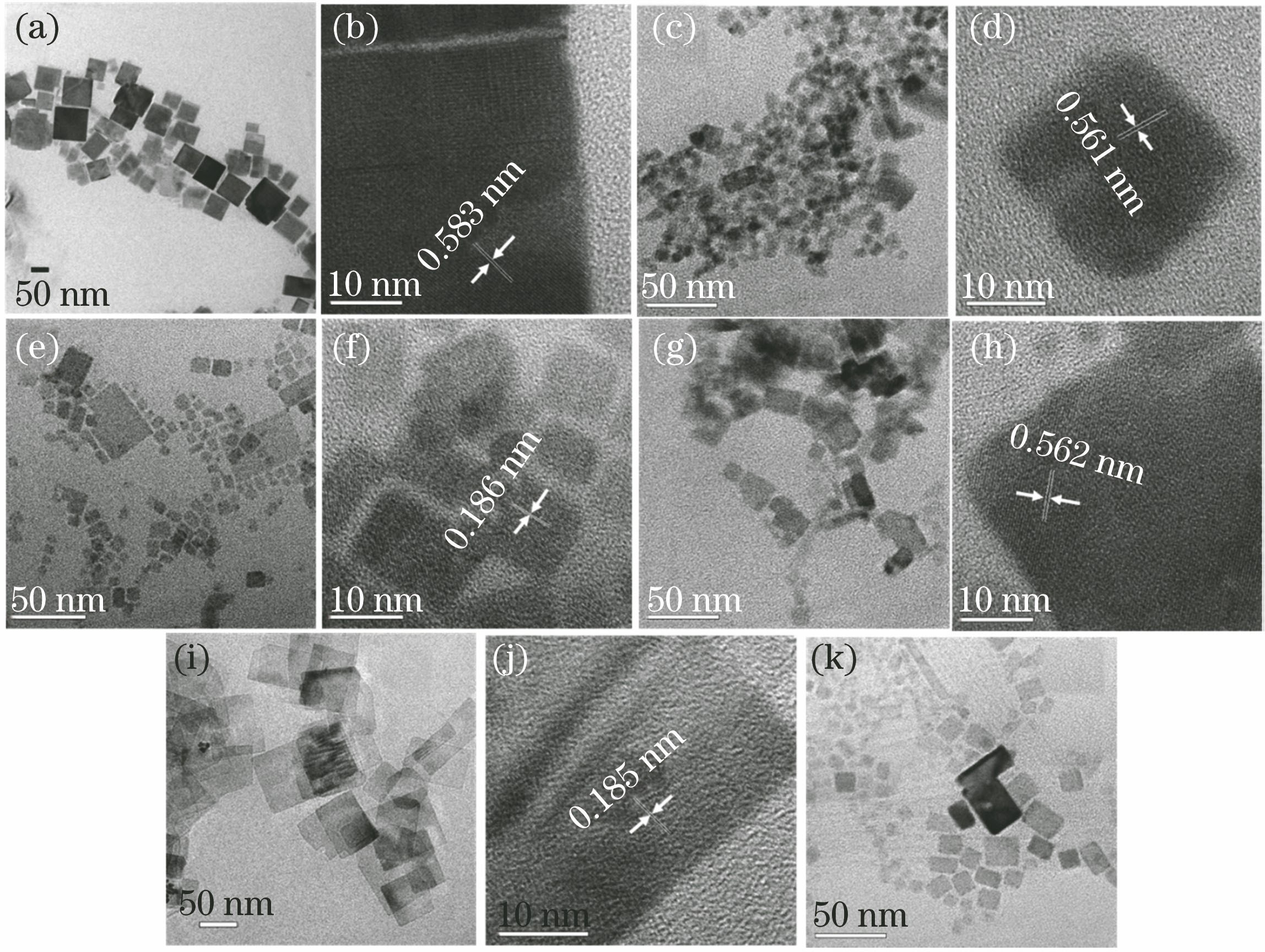
Fig. 2. TEM and HR-TEM images of nanocrystals. (a) TEM image of CsPbBr3; (b) HR-TEM image of CsPbBr3; (c) TEM image of CsPbCl3 prepared using FeCl3 as chlorine source; (d) HR-TEM image of CsPbCl3 prepared using FeCl3 as chlorine source; (e) TEM image of CsPbCl3 prepared using FeCl2 as chlorine source; (f) HR-TEM image of CsPbCl3 prepared using FeCl2 as chlorine source; (g) TEM image of CsPbCl3 prepared using CoCl2 as chlorine source; (h) HR-TEM image of CsPbCl3 prepared using CoCl2 as chlorine source; (
Fig. 3. UV-vis absorption spectrum, emission spectrum and digital photographs of CsPbBr3 nanocrystals. (a) UV-vis absorption and emission spectra; (b) digital photograph without UV light irradiation; (c) digital photograph with 365 nm UV light irradiation
Fig. 4. UV-vis absorption spectra, emission spectra and digital photographs of as-obtained nanocrystals using different chlorine sources. (a) UV-vis absorption spectra; (b) emission spectra; (c) digital photographs without UV light irradiation (from left to right, CoCl2, FeCl2, FeCl3, and NiCl2 used as chlorine sources); (d) digital photographs with 365 nm UV light irradiation (from left to right, CoCl2, FeCl2, FeCl3, and NiCl2 used as chlorine sources)
|
Table 1. Chemical compositions of samples (atomic fraction, %)

Set citation alerts for the article
Please enter your email address



