- Journal of Inorganic Materials
- Vol. 36, Issue 2, 161 (2021)
Abstract
Electrochromic devices (ECDs) can reversibly change their light transmittance via external voltage-induced redox reactions, and show potential applications in smart windows, electrochromic displays, switching mirrors, sensors, supercapacitors and so on[
To overcome the above mentioned problems, gel polymer electrolytes (GPEs) have been developed; these GPEs generally show a much higher conductivity than solid polymer electrolytes (> 10-4vs 10-8 S∙cm-1)[
It is well accepted that fillers play an important role in Li+ transport, but the apparent discrepancy in electrochemical performances can partially be ascribed to the difference in electrolyte materials (polymers, salt, filler type), along with their concentration and preparation conditions. The addition of nanoparticles (such as TiO2[
Fumed SiO2 has been widely adopted as a filler to improve the conductivity and mechanical stability of GPEs, because of its branched three-dimensional structure and the flexibly-tunable surface functionalities[
In this study, we investigate the effect of a hydrophobic fumed SiO2/PMMA/PC/LiClO4 GPE (H-SiO2 GPE) and their applications in ECDs. It is demonstrated that hydrophobic fumed SiO2 is more efficient in increasing the ionic conductivity of electrolytes, because of the hydrophobic- hydrophobic attractions between the hydrophobic groups on the SiO2 surfaces and solvents in the GPE.The ECD assembled with H-SiO2 GPE exhibits a fast switching speed (tbleaching=4 vs 8 s and tcoloring=14 vs 16 s). To compare, the effect of a hydrophobic fumed SiO2/LiClO4/ PC/liquid electrolyte is explored. This work demonstrates that the addition of hydrophobic fumed SiO2 in electrolytes provides great potential for the application in ECDs and other energy devices.
1 Experimental
1.1 Chemicals
Propylene carbonate (PC, 99%), lithium perchlorate (LiClO4, 99.9%), hydrophobic fumed SiO2 (Aladdin, 7-40 nm, pH=4.5), polymethyl methacrylate (PMMA, Aldrich, Mw≈1000, 000) were obtained and used.
1.2 Preparation of the liquid electrolytes and GPEs
The liquid electrolyte was prepared by dissolving 0.1 mol·L-1 LiClO4 in 7.41 mL propylene carbonate under stirring. Afterward, 0, 20, 50, 80 and 100 mg of hydrophobic fumed SiO2 was dispersed into 10 g of liquid electrolyte with ultrasonication. This process provided hydrophobic fumed SiO2/LiClO4/PC liquid electrolyte with 0, 0.2wt%, 0.5wt%, 0.8wt% and 1.0wt% fumed SiO2. Hydrophobic fumed SiO2/polymethyl methacrylate (PMMA)/PC/LiClO4 GPEs (H-SiO2 GPEs) were prepared by dissolving 1 g of PMMA (vacuum dried at 90 ℃ for 24 h) into 9 g of liquid electrolyte under stirring at 90 ℃.
1.3 Preparation of the WO3 nanopowder
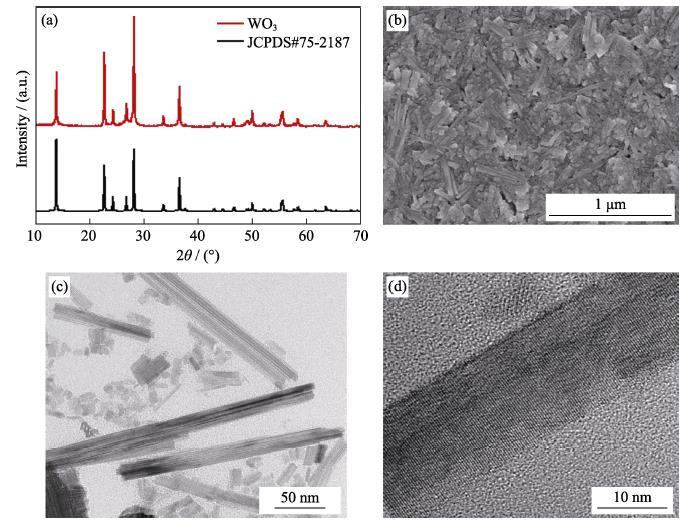
WO3 nanoparticles were synthesized using the reported hydrothermal process[
1.4 Assembly of ECDs based on different electrolytes
WO3 electrochromic films were deposited on clean ITO glasses by a wire-bar coating method, as reported in our previous work[
1.5 Characterization
Viscosity measurements were determined by rotary rheometer (HAAKE MARS60) under shear rate of 0.1- 100 s-1 at room temperature. The morphology of WO3 films was characterized by scanning electron microscope (SEM, S-4800, Hitachi, Tokyo, Japan) and transmission electron microscope (TEM, JEOL, JEM-2100, 200 kV). The morphologies of the gel electrolyte were characterized by FE-SEM (JEOL, JSM6700F, Tokyo). The crystal structure of the electrochromic film was required by X-ray diffraction (XRD; 3kW Bruker D8 Advance X-ray diffractometer with Cu Kα irradiation, λ=0.154 nm). The optical transmittance of the electrochromic devices was characterized by UV spectrophotometer (UH4150; Hitachi, Japan) at 633 nm. The ionic conductivity was measured by Mettler Seven compact conductivity meter. Electrochemical measurements were performed on electrochemical workstation (CHI760E, CHI Instruments, China). Electrochemical impedance spectroscopy (EIS) of the electrochromic film was recorded from 10-1 to 106 Hz at an amplitude of 5 mV.
2 Results and discussion
2.1 Phase characterization
The hydrophobicity of SiO2 is investigated by the contact angle test, as presented in Fig. S1. Regardless of how the needle moves, water droplets cannot adhere to the surface of hydrophobic fumed SiO2. Fig. 1 shows the FT-IR spectrum of the hydrophobic fumed SiO2. The broad peaks at 3446 and 1631 cm-1 are the -OH stretching and bending vibration peaks of adsorbed water, respectively. The peaks at 808 and 478 cm-1 are the symmetric stretching vibrations and bending vibrations of Si-O bonds. The strong and wide absorption band at 1110 cm-1 is the anti- symmetric stretching vibration peak of Si-O-Si. The peak at 979 cm-1 is the stretching vibration of Si-OH, indicating the possibility for further reaction as a proton donor[
![]()
Figure 1.Static water contact angle (CA) of hydrophobic fumed SiO2
![]()
Figure 2.Nyquist plots (a), ionic conductivity (b) and viscosity (c) of PMMA-based GPEs with 0, 0.2wt%, 0.5wt%, 0.8wt% and 1.0wt% fumed SiO2. Electrochromic switching behaviors at 633 nm (e), colored state EIS (d) and CV curves (f) of ECDs contained PMMA-based GPEs with 0, 0.5wt% fumed SiO2. Scan rate: 100 mV/s
2.2.2 Electrochromic performances of ECDs based on H-SiO2 GPEs
2.2 Effect of fumed SiO2 on the Gel electrolyte and electrochromic devices
To explore the effect of H-SiO2 GPEs on electrochromism, the ECDs were assembled by WO3 coated on ITO glass[
![]()
Figure .Ionic conductivity of liquid electrolyte with 0, 0.2wt%, 0.5wt%, 0.8wt%, and 1.0wt% fumed SiO2 (a), and colored state Nyquist plots (b) and CV curves (c) of WO3 films in liquid electrolyte with 0, 0.2wt%, 0.5wt%, 0.8wt% and 1.0wt% fumed SiO2. Scan rate: 100 mV/s
2.2.1 Measurements of the ionic conductivities of H-SiO2 GPEs
![]()
Figure 2.Nyquist plots (a), ionic conductivity (b) and viscosity (c) of PMMA-based GPEs with 0, 0.2wt%, 0.5wt%, 0.8wt% and 1.0wt% fumed SiO2. Electrochromic switching behaviors at 633 nm (e), colored state EIS (d) and CV curves (f) of ECDs contained PMMA-based GPEs with 0, 0.5wt% fumed SiO2. Scan rate: 100 mV/s
A high ionic conductivity facilitates the transportation of ions into and out of an EC film to initiate a color change, which is the key indicator of an electrolyte. The ionic conductivities of these GPEs are calculated by the formula σ = L/RbA, where L is the thickness of the GPE electrolyte, A is the contact area between the electrolyte and the electrode, and the bulk resistance (Rb) is the intercept of the curve and the Z’ axis in the AC impedances (Fig. 2(a))[
![]()
Figure 1.Static water contact angle (CA) of hydrophobic fumed SiO2
To further investigate the electrochromic performances of ECDs, as shown in Fig. 2(e). An ECD with H-SiO2 GPEs switches faster than that without SiO2. More precisely, the switching time sare 16/14 s (coloring) and 8/4s (bleaching) for ECDs with pure GPEs and H-SiO2 GPEs, respectively. Compared with other similar constructions of ECDs (using WO3, ITO glass and a gel electrolyte as electrochromic layers, substrates and ion transport layers, respectively), H-SiO2 GPEs exhibit faster speeds than previously reported results for PVdF- HFP-based (tcoloring=17 s/tbleaching=28 s)[
![]()
Figure 3.FT-IR spectrum of hydrophobic fumed SiO2
| Electrolyte | Ionic conductivity/(mS·cm-1) |
|---|---|
| PMMA/LiClO4/hydrophobic | 5.14 |
| PMMA/LiClO4/hydrophilic SiO2[ | 3.8 |
| P(BMA-St)/hydrophilic SiO2[ | 2.15 |
| PEO/LiCF3SO3/TiO2[ | 0.16 |
| PVDF/LiClO4/palygorskite[ | 0.12 |
| PMMA/LiClO4/[Emim]BF4[ | 2.9 |
| PVB/LiClO4[ | 0.04 |
| PVDF-HFP/LiCF3SO3/ZrO2[ | 1.78 |
| PAN/LiClO4/Li0.33La0.557TiO3[ | 0.0605 |
Table 1.
Ion conductivity of gel electrolytes from the literature
In addition, the CV curves of the ECDs with different GPEs are shown in Fig. 2(f). The CV curve of the SiO2-free ECD has a cathode current peak at -3.3 V and an anodic current peak at 1.3 V. In contrast, the CV curves of the SiO2 containing ECDs show cathode current peaks at +0.37 V, and anodic current peaks at -2.6 V. These new peaks may be attributed to different binding sites on the WO3 film[
H-SiO2 GPEs are shown in Fig. S3. H-SiO2 evenly distributes in the main body of the GPEs.
![]()
Figure 3.FT-IR spectrum of hydrophobic fumed SiO2
2.3 Effect of fumed SiO2 on the liquid electrolyte and electrochromic devices
For a LiClO4/PC liquid electrolyte, the PC solvent has a strong solvation effect on Li+ cations, but the perchlorate anions are difficult to coordinate or solvate, limiting the achievement of high conductivity in this system. The conductivity of liquid electrolytes was measured by a conductivity meter, and the results show a similar trend to that of gel electrolytes. To increase the accuracy of data, the experiment was repeated three times (Fig. 4(a)). With SiO2 additives from 0 to 0.5wt%, the ionic conductivity of the liquid electrolyte increases up to a maximum of 7.58 mS∙cm-1, which is higher than hydrophilic fumed SiO2 or other additives as fillers (no more than 7.0 mS∙cm-1)[
![]()
Figure .Ionic conductivity of liquid electrolyte with 0, 0.2wt%, 0.5wt%, 0.8wt%, and 1.0wt% fumed SiO2 (a), and colored state Nyquist plots (b) and CV curves (c) of WO3 films in liquid electrolyte with 0, 0.2wt%, 0.5wt%, 0.8wt% and 1.0wt% fumed SiO2. Scan rate: 100 mV/s
The effects of the hydrophobic fumed SiO2 composite electrolyte on the electrochemical behaviors of WO3 films were characterized by electrochemical impedance spectroscopy in the colored state (EIS, Fig. 4(b)). As shown in Fig. 4(b), both the charge transfer resistance and ion diffusion resistance reach a minimum at a SiO2 concentration of 0.5wt%, which is consistent with the conductivity variation. Fig. 4(c) further presents the CV curves of the WO3 films in SiO2 electrolytes at different concentrations. In all of CV curves, clear cathodic current peaks appear with potential sweeping from +1 V to -1 V. Moreover, the colorless WO3 film appears blue, due to the reduction of W6+→W5+ after Li+ insertion. The film returns to be colorless after reverse sweeping the potential back to +1 V, suggesting the occurrence of the W5+→W6+ oxidation reaction accompanied by Li+ extraction. Therefore, the WO3 film demonstrates the largest CV curve in the highly conductive electrolyte containing the addition of 0.5wt% fumed SiO2, confirming the beneficial influence of the fumed SiO2 additive on the electrochemical reactions of the WO3 film.
3 Conclusion
In conclusion, the introduction of hydrophobic fumed SiO2 into GPEs can effectively increase the conductivity from 3.99 to 5.14 mS∙cm-1, because of the attractive force of hydrophobic groups. These interactions are beneficial for increasing the compatibility between SiO2 and GPEs, thereby promoting the dissociation of lithium perchlorate, and improving the ionic conductivity. The assembled ECDs using H-SiO2 GPEs exhibit lower cathode interface impedances, better electrochemical behaviors, and faster switching times, as compared with those of the unmodified GPEs (tbleaching=4 vs 8 s and tcoloring=14 vs 16 s). These observations are owing to that H-SiO2 GPEs form a three-dimensional network structure, thereby providing an ion transport channel. Similarly, the ionic conductivity of the LiClO4/PC/liquid electrolyte without/with hydrophobic fumed SiO2 increases from 6.94 mS∙cm-1 (without hydrophobic fumed SiO2) to 7.58 mS∙cm-1. This work demonstrates that the introduction of hydrophobic SiO2 has a positive effect on electrolytes, which is an important step for their application in ECDs.
Supporting materials:
ZHAO Qi1, QIAO Ke1, YAO Yongji1, CHEN Zhang1, CHEN Dongchu2, GAO Yanfeng1
1. School of Materials Science and Engineering, Shanghai University, Shanghai 200444, China; 2. School of Materials Science and Energy Engineering, Foshan University, Foshan 528000, China
References:
[1] AHMAD S, AHMAD S, AGNIHOTRY S A. Nanocomposite electrolytes with fumed silica in poly(methyl methacrylate): thermal, rheological and conductivity studies. Journal of Power Sources, 2005, 140: 151-156.
[2] LIAO Y H, RAO M M, LI W S, et al. Fumed silica-doped poly(butyl methacrylate-styrene)-based gel polymer electrolyte for lithium ion battery. Journal of Membrane Science, 2010, 352: 95-99.
[3] VIGNAROOBAN K, DISSANAYAKE M A K L, ALBINSSON I, et al. Effect of TiO2 nano-filler and EC plasticizer on electrical and thermal properties of poly(ethylene oxide) (PEO) based solid polymer electrolytes. Solid State Ionics, 2014, 266: 25-28.
[4] YAO P, ZHU B, ZHAI H, et al. PVDF/palygorskite nanowire composite electrolyte for 4 V rechargeable lithium batteries with high energy density. Nano Letters, 2018, 18: 6113-6120.
[5] TANG Q, LI H, YUE Y, et al. 1-Ethyl-3-methylimidazolium tetrafluoroborate-doped high ionic conductivity gel electrolytes with reduced anodic reaction potentials for electrochromic devices. Materials & Design, 2017, 118: 279-285.
[6] ZHANG F, DONG G, LIU J, et al. Polyvinyl butyral-based gel polymer electrolyte films for solid-state laminated electrochromic devices. Ionics, 2017, 23: 1879-1888.
[7] PUGUAN J M C, CHINNAPPAN A, APPIAH-NTIAMOAH R, et al. Enhanced ionic conductivity and optical transmissivity of functionalized ZrO2/PVdF-HFP hybrid electrolyte for energy efficient windows. Solar Energy Materials and Solar Cells, 2015, 137: 265-273.
[8] LIU W, LEE S W, LIN D. Enhancing ionic conductivity in composite polymer electrolytes with well-aligned ceramic nanowires. Nature Energy, 2017, 2(5): 17035.
References
[1] J PATEL K, G BHATT G, R RAY J et al. All-inorganic solid-state electrochromic devices: a review. Journal of Solid State Electrochemistry, 21, 1-11(2016).
[2] SA AGNIHOTRYA, SS SEKHON P. PMMA based gel electrolyte for EC smart windows. Electrochimica Acta, 44, 3121-3126(1998).
[3] H LI, J WANG, Q SHI et al. Constructing three-dimensional quasi- vertical nanosheet architectures from self-assemble two-dimensional WO3·2H2O for efficient electrochromic devices. Applied Surface Science, 380, 281-287(2016).
[4] F GROCE, B APPETECCHI G, L PERSI et al. Nanocomposite polymer electrolytes for lithium batteries. Nature, 394, 456(1998).
[5] B AZIZ S, J WOO T, Z KADIR M F et al. A conceptual review on polymer electrolytes and ion transport models. Journal of Science: Advanced Materials and Devices, 3, 1-17(2018).
[6] C TAO, M GAO, B YIN et al. A promising TPU/PEO blend polymer electrolyte for all-solid-state lithium ion batteries. Electrochimica Acta, 257, 31-39(2017).
[7] R JUNG H, H JU D, J LEE W et al. Electrospun hydrophilic fumed silica/polyacrylonitrile nanofiber-based composite electrolyte membranes. Electrochimica Acta, 54, 3630-3637(2009).
[8] V KUPPU S, R JEYARAMAN A, K GURUVIAH P et al. Preparation and characterizations of PMMA-PVDF based polymer composite electrolyte materials for dye sensitized solar cell. Current Applied Physics, 18, 619-625(2018).
[9] W ZHAI, H ZHU, L WANG et al. Study of PVDF-HFP/PMMA blended micro-porous gel polymer electrolyte incorporating ionic liquid [BMIM] BF4 for lithium ion batteries. Electrochimica Acta, 133, 623-630(2014).
[10] K VIGNAROOBAN et al. Effect of TiO2 nano-filler and EC plasticizer on electrical and thermal properties of poly(ethylene oxide)(PEO) based solid polymer electrolytes. Solid State Ionics, 266, 25-28(2014).
[11] G VIJAYAKUMAR, N KARTHICK S et al. Effect of nanoscale CeO2 on PVDF-HFP-based nanocomposite porous polymer electrolytes for Li-ion batteries. Journal of Solid State Electrochemistry, 12, 1135-1141(2007).
[12] F CROCE, B SCROSATI, G MARIOTTO. Electrochemical and spectroscopic study of the transport properties of composite polymer electrolytes. Chem. Mater., 4, 1134-1136(1992).
[13] P YAO, B ZHU, H ZHAI et al. PVDF/palygorskite nanowire composite electrolyte for 4 V rechargeable lithium batteries with high energy density. Nano Letters, 18, 6113-6120(2018).
[14] S AHMAD, S AHMAD, A AGNIHOTRY S. Nanocomposite electrolytes with fumed silica in poly(methyl methacrylate): thermal, rheological and conductivity studies. Journal of Power Sources, 140, 151-156(2005).
[15] M JITJAICHAM, B KUSUKTHAM. Spinning of poly(ethylene terephthalate) fiber composites incorporated with fumed silica. Silicon, 10, 575-583(2017).
[16] J WANG, E KHOO, PS LEE et al. Synthesis, assembly, and electrochromic properties of uniform crystalline WO3 nanorods. J. Phys. Chem. C, 112, 14306-14312(2008).
[17] Q ZHAO, Y FANG, K QIAO et al. Printing of WO3/ITO nanocomposite electrochromic smart windows. Solar Energy Materials and Solar Cells, 194, 95-102(2019).
[18] A EL-FATTAH M, A El SAEED. Chemical interaction of different sized fumed silica with epoxy
[19] C PUGUAN J M, J CHUNG W, H KIM et al. Ion-conductive and transparent PVdF-HFP/silane-functionalized ZrO2 nanocomposite electrolyte for electrochromic applications. Electrochimica Acta, 196, 236-244(2016).
[20] D SAIKIA, G WU C, J FANG et al. Organic-inorganic hybrid polymer electrolytes based on polyether diamine, alkoxysilane, and trichlorotriazine: synthesis, characterization, and electrochemical applications. Journal of Power Sources, 269, 651-660(2014).
[21] Q TANG, H LI, Y YUE et al. 1-Ethyl-3-methylimidazolium tetrafluoroborate-doped high ionic conductivity gel electrolytes with reduced anodic reaction potentials for electrochromic devices. Materials & Design, 118, 279-285(2017).
[22] R LEONES, C SABADINI R, C SENTANIN F et al. Polymer electrolytes for electrochromic devices through solvent casting and Sol-Gel routes. Solar Energy Materials & Solar Cells, 169, 98-106(2017).
[23] F ZHANG, G DONG, J LIU et al. Polyvinyl butyral-based gel polymer electrolyte films for solid-state laminated electrochromic devices. Ionics, 23, 1879-1888(2017).
[24] R RAGHAVAN S, W RILEY M, S FEDKIW P et al. Composite polymer electrolytes based on poly(ethylene glycol) and hydrophobic fumed silica: dynamic rheology and microstructure. Chemistry of Materials, 10, 244-251(1998).
[25] A ŠURCA VUK, V JOVANOVSKI, A POLLET-VILLARD et al. Imidazolium-based ionic liquid derivatives for application in electrochromic devices. Solar Energy Materials and Solar Cells, 92, 126-135(2008).
[26] J BAE, H KIM, C MOON H et al. Low-voltage, simple WO3-based electrochromic devices by directly incorporating an anodic species into the electrolyte. Journal of Materials Chemistry C, 46, 10887-10892(2016).
[27] J CUI, Z ZHOU, M JIA et al. Solid polymer electrolytes with flexible framework of SiO2 nanofibers for highly safe solid lithium batteries. Polymers, 12, 1324(2020).
Set citation alerts for the article
Please enter your email address



