Jun-hang DONG, Zhen-li ZHU, Han-qing DING, Peng-ju XING, Fei-yang ZHOU, Hong-tao ZHENG, Xing LIU. Research Progress of Isotope Analysis Method Based on Optical Spectroscopy[J]. Spectroscopy and Spectral Analysis, 2022, 42(8): 2325
Search by keywords or author
- Spectroscopy and Spectral Analysis
- Vol. 42, Issue 8, 2325 (2022)
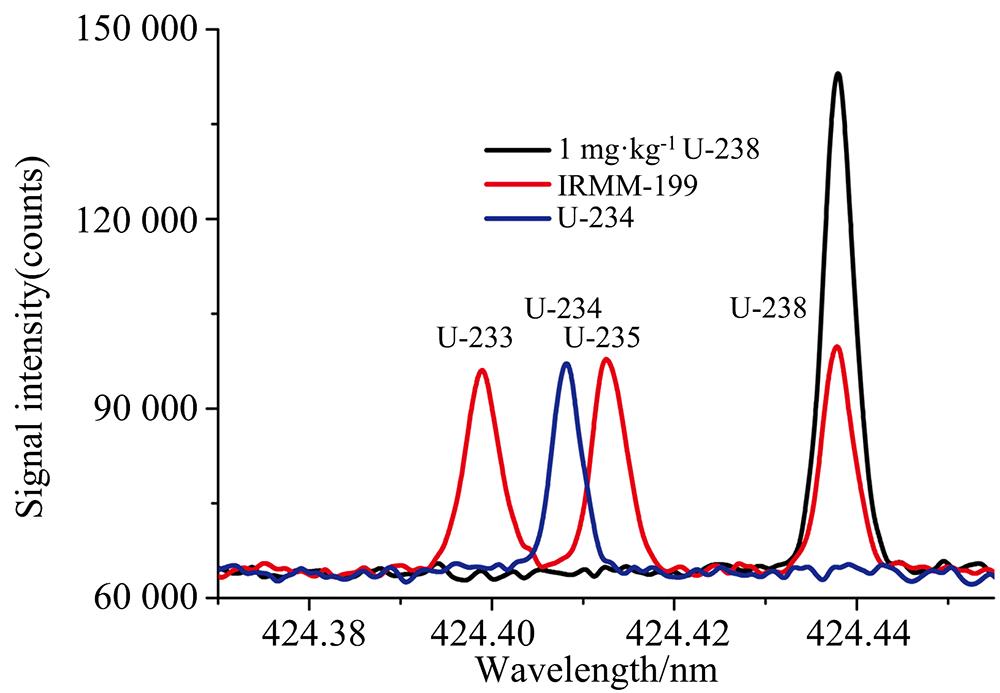
![233U, 235U and 238U emission spectra in the 424.37~424.45 nm wavelength regions for the certified isotopic reference material IRMM-199 containing ~0.45 mg·kg-1 each of 233U, 235U, and 238U as well as a conventional U single-element standard solution (238U and 234U) are also additionally displayed. 233U (424.398 nm), 235U (424.412 nm) and 238U (424.437 nm) can be clearly distinguished, and 234U can also be effectively identified[11]](/richHtml/gpxygpfx/2022/42/8/2325/img_1.png)
Fig. 1. 233U, 235U and 238U emission spectra in the 424.37~424.45 nm wavelength regions for the certified isotopic reference material IRMM-199 containing ~0.45 mg·kg-1 each of 233U, 235U, and 238U as well as a conventional U single-element standard solution (238U and 234U) are also additionally displayed. 233U (424.398 nm), 235U (424.412 nm) and 238U (424.437 nm) can be clearly distinguished, and 234U can also be effectively identified[11]
![Emission spectra of (a) atomic carbon emission line, (b) molecular isotopic band during LAMIS of carbon isotope sampleSpectrometer resolution, gate delay and width were 0.02 nm, 4 μs and 2 μs, respectively[25]](/richHtml/gpxygpfx/2022/42/8/2325/img_2.png)
Fig. 2. Emission spectra of (a) atomic carbon emission line, (b) molecular isotopic band during LAMIS of carbon isotope sample
Spectrometer resolution, gate delay and width were 0.02 nm, 4 μs and 2 μs, respectively[25]
Spectrometer resolution, gate delay and width were 0.02 nm, 4 μs and 2 μs, respectively[25]
Fig. 3. LA-TDLAS spectra (6Li and 7Li) at delay time of 340 μs after ablation pulse[43]
Fig. 4. Z-AAS determination of (a) 198Hg, 200Hg, 201Hg, and 202Hg isotopes (symbols) and the simulation results (solid lines) and (b) mixed isotopes of 200Hg and 198Hg and the natural Hg[8]
Fig. 5. Absorption of 14N and 15N-isotopes of N2O centered at ~2 188.688, ~2 188.756 and ~2 188.938 cm-1, respectively
The measured data (green points) and fitted curves (red lines) are shown in the upper trace and the residuals (blue lines) in the lower trace[52]
The measured data (green points) and fitted curves (red lines) are shown in the upper trace and the residuals (blue lines) in the lower trace[52]
Fig. 6. Spectra of AlCl (1:1, 35Cl/37Cl molar ratio solution) at wavelengths of: (a) 261.418 nm; (b) 261.695 nm; (c) 261.819 nm; (d) 262.238 nm; (e) 262.697 nm; (f) 263.216 nm; (g) 263.807 nm; and (h) 264.490 nm
They were obtained for 400 ng of Cl, 10 mg of Al and 20 mg of Pd. However, B shows two spectraof AlCl with different isotope abundance: in black, the one obtained for 400 ng of Cl of CRM NIST 975a (35Cl, 75.774%), and in red, the one obtained for 400 ng of Cl of CRM AE642 (37Cl, 98.122%)[53]
They were obtained for 400 ng of Cl, 10 mg of Al and 20 mg of Pd. However, B shows two spectraof AlCl with different isotope abundance: in black, the one obtained for 400 ng of Cl of CRM NIST 975a (35Cl, 75.774%), and in red, the one obtained for 400 ng of Cl of CRM AE642 (37Cl, 98.122%)[53]
|
Table 1. Isotope analysis methods for optical emission spectroscopy
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Table 2. Isotope analysis methods by LAMIS
Set citation alerts for the article
Please enter your email address



