Weiwei Chen, Jianrong Qiu, Guoping Dong. Research progress on ultra-broadband luminescence of Bi-doped glass and fiber (invited)[J]. Infrared and Laser Engineering, 2023, 52(5): 20230097
Search by keywords or author
- Infrared and Laser Engineering
- Vol. 52, Issue 5, 20230097 (2023)
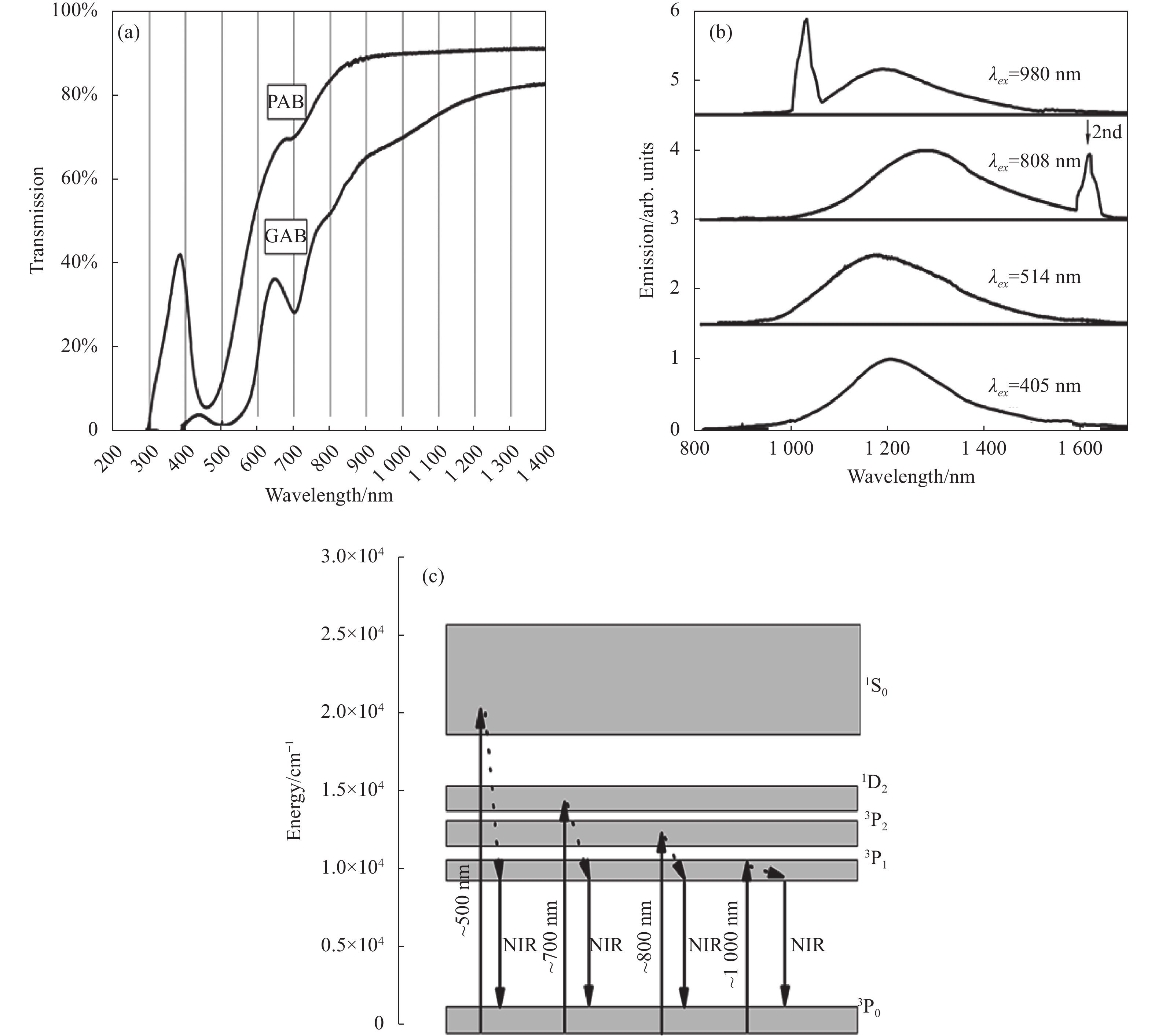
![(a) Transmission spectra of Bi-doped phosphate glass; (b) Normalized emission spectra of Bi-doped phosphate glass excited by 405, 514, 808, and 980 nm, respectively; (c) Energy level diagram for Bi+ based on energy matching conditions[8]](/richHtml/irla/2023/52/5/20230097/img_1.jpg)
Fig. 1. (a) Transmission spectra of Bi-doped phosphate glass; (b) Normalized emission spectra of Bi-doped phosphate glass excited by 405, 514, 808, and 980 nm, respectively; (c) Energy level diagram for Bi+ based on energy matching conditions[8]
![Calculated the differential charge density of interstitial Bi0 atom in 60-atom silica cluster model. (a) Map plane goes through Bi atom and two nearest Si atoms; (b) Map plane goes through Bi atom and two nearest O atoms; (c) Defect-free 96-atom supercell of fused silica model; (d) Interstitial Bi0 atom in 60-atom silica cluster model (Si atom is in gold, O atom is in red, Bi atom is in violet, and H atom is in white); (e) Calculated energy levels diagram of interstitial Bi0 atom in silica optical fiber, which are responsible for the NIR emission[11]](/richHtml/irla/2023/52/5/20230097/img_2.jpg)
Fig. 2. Calculated the differential charge density of interstitial Bi0 atom in 60-atom silica cluster model. (a) Map plane goes through Bi atom and two nearest Si atoms; (b) Map plane goes through Bi atom and two nearest O atoms; (c) Defect-free 96-atom supercell of fused silica model; (d) Interstitial Bi0 atom in 60-atom silica cluster model (Si atom is in gold, O atom is in red, Bi atom is in violet, and H atom is in white); (e) Calculated energy levels diagram of interstitial Bi0 atom in silica optical fiber, which are responsible for the NIR emission[11]
Fig. 3. (a) Transmittance spectrum of Bi-doped silicate glass; (b)-(d) Emission spectra of Bi-doped silicate glass under excitation at 500 nm, 700 nm, and 800 nm, respectively[1]
Fig. 4. (a) Effect content of Al2O3 content on the NIR emission spectra of Bi-doped germanate glasses[35]; (b) Effect of GeO2 content on the NIR emission spectra of Bi-doped borate glasses[38]; (c) Effect of GeO2 content on the NIR emission spectra of Bi-doped silicate glasses[39]; (d) Effect of CaO content on the NIR emission spectra of Bi-doped borate glasses[41]
Fig. 5. (a) NIR emission spectra of Bi-doped borate glasses with different carbon content (λ ex = 450 nm), the inserts are the images of glass samples[42]; (b) NIR emission spectra of samples PGB1 and PGB3-6 pumped by 980 nm treated under different atmosphere[43]; (c) Comparison between the emission spectra of the Bi single doped and Bi-AlN co-doped glass samples (λ ex = 467 nm) [44]; (d) NIR emission spectra (λex = 468 nm) of Bi-doped Nx samples (x = 0-0.1 mol%) with Si3N4 content varying[45]
Fig. 6. (a) Optical microscope image of grating under 6.0 μJ of fs laser pulse energy, and photographs of various sample irradiated under different pulse energy (0-8.0 μJ, as labeled); (b) Absorption and (c) NIR emission spectra (λ ex = 808 nm) of the glass samples under different fs laser pulse energy[51]
Fig. 7. (a) NIR emission spectra of Bi, Er single-doped glass, and Bi-Er co-doped glass under the excitation of 808 nm LD[52]; (b) NIR emission spectra of Bi, Er, Nd single-doped glass, and Bi-Er-Nd co-doped glass under the excitation of 808 nm LD[53]; (c) NIR emission spectra of Bi, Er, Tm single-doped glasses under the excitation of 808 nm LD; (d) NIR emission spectra of Bi-Er-Tm co-doped glass under the excitation of 808 nm LD[54]
Fig. 8. (a) Normalized emission spectra at emission peak ~1140 nm of Bi‐doped borate glass samples with elevating GeO2 content[33]; (b) Comparison between the emission spectra of Bi-doped germanate glass samples 0 SiC and 3 SiC by Gauss fitting[56]; (c) Comparison of the emission spectra of Bi-doped germanate glass samples without AlN (N0B0.02), with AlN (N2B0.02), and with 3 mol% Bi (N0B3)[55]
Fig. 9. (a) Schematic diagram of Bi-doped fiber preform prepared by MCVD method[60]; (b) Sum diagram of the major luminescence range and lifetime of various Bi-doped optical fibers[61]
Fig. 10. (a) Schematic representation of the optical fibers fabricated by the molten core method[65-66]; (b) Optical image of Bi-doped fiber cross-section fabricated by the molten core method and EPMA images of fiber cross-section[67]
Fig. 11. (a) Preparation process of Bi-doped fiber by the rod-in-tube technique; (b) EPMA-WDS mappings of different elements from the Bi-doped fiber cross section[55]
Fig. 12. Output power, peak position and corresponding excitation wavelength of Bi doped fiber laser[86]
|
Set citation alerts for the article
Please enter your email address



