Ya TANG, Shengrui SUN, Jia FAN, Qingfeng YANG, Manjiang DONG, Jiahui KOU, Yangqiao LIU. PEI Modified Hydrated Calcium Silicate Derived from Fly Ash and Its adsorption for Removal of Cu (II) and Catalytic Degradation of Organic Pollutants [J]. Journal of Inorganic Materials, 2023, 38(11): 1281
Search by keywords or author
- Journal of Inorganic Materials
- Vol. 38, Issue 11, 1281 (2023)
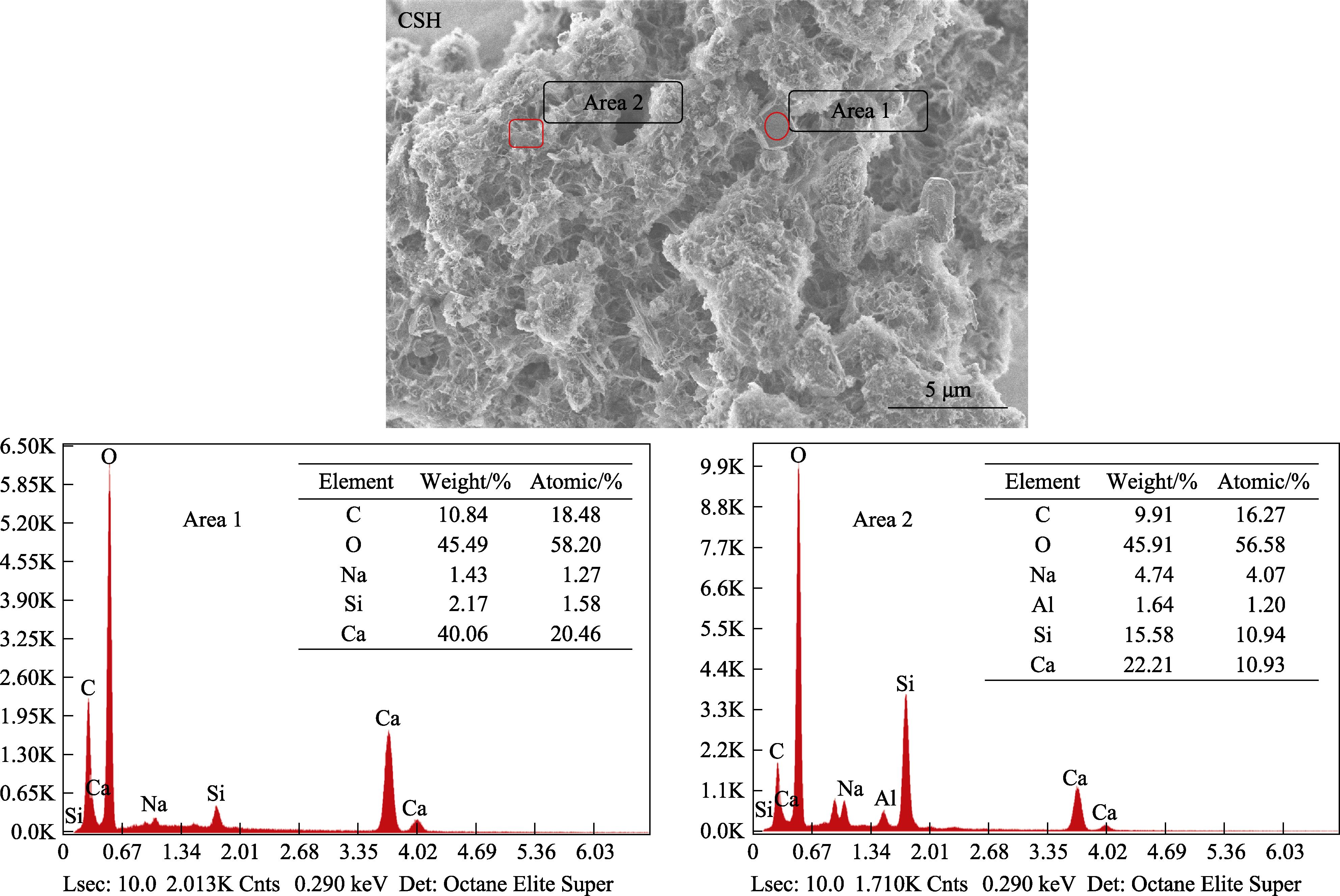
S1. EDS analyses of CSH

1. (a) XRD patterns, (b) FT-IR spectra and (c) N2 adsorption-desorption isotherms of CSH and PCSH
2. Different magnification SEM images of (a, b) CSH and (c, d) PCSH
S2. EDS analysis of PCSH
S3. Linear fitting curves of (a) Langmuir model and (b) Freundlich model for isotherms of CSH and PCSH
3. Adsorption characteristics of samples
S4. EDS analyses of PCSH-Cu
4. Characteristics of Cu-absorbed samples
5. SEM images of (a) CSH-Cu, (b) PCSH-Cu, (c) CSH- Cu-c, and (d) PCSH-Cu-c
6. (a) XRD patterns and (b) FT-IR spectra of CSH-Cu-c and PCSH-Cu-c
7. Degradation of RhB by PMS with CSH-Cu-c and PCSH-Cu-c
8. Suface elements and chemical status of samples
9. Free radical capture experiments of PCSH-Cu-c
10. Change of 4-NP residue percentage
|
Table 1. Rate constants (k) for the degradation of RhB by PMS with different catalysts
| |||||||||||||||||||||||||||
Table 1. Langmuir and Freundlich isotherm fitting parameters
|
Table 2. Comparison of maximum adsorption capacities of various sorbents as reported in the literature for Cu(II)
|
Table 2. Rate constants (k) for the degradation of 4-NP by NaBH4 with different catalysts

Set citation alerts for the article
Please enter your email address



