Hongli WANG, Nan WANG, Liying WANG, Erhong SONG, Zhankui ZHAO. Hydrogen Generation from Formic Acid Boosted by Functionalized Graphene Supported AuPd Nanocatalysts [J]. Journal of Inorganic Materials, 2022, 37(5): 547
Search by keywords or author
- Journal of Inorganic Materials
- Vol. 37, Issue 5, 547 (2022)
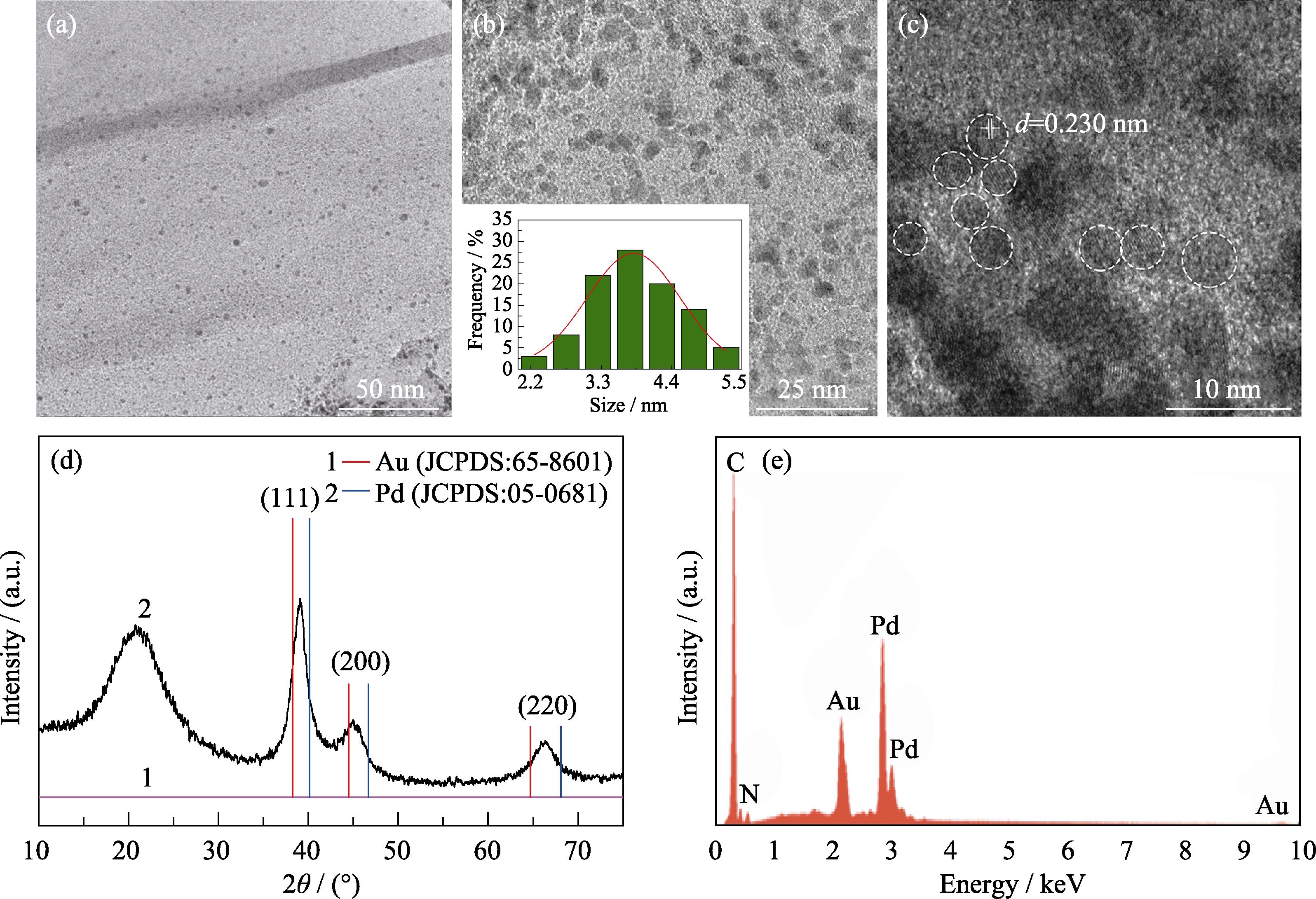
2. TEM images, XRD pattern and EDX spectrum of Au0.3Pd0.7/PEI-rGO
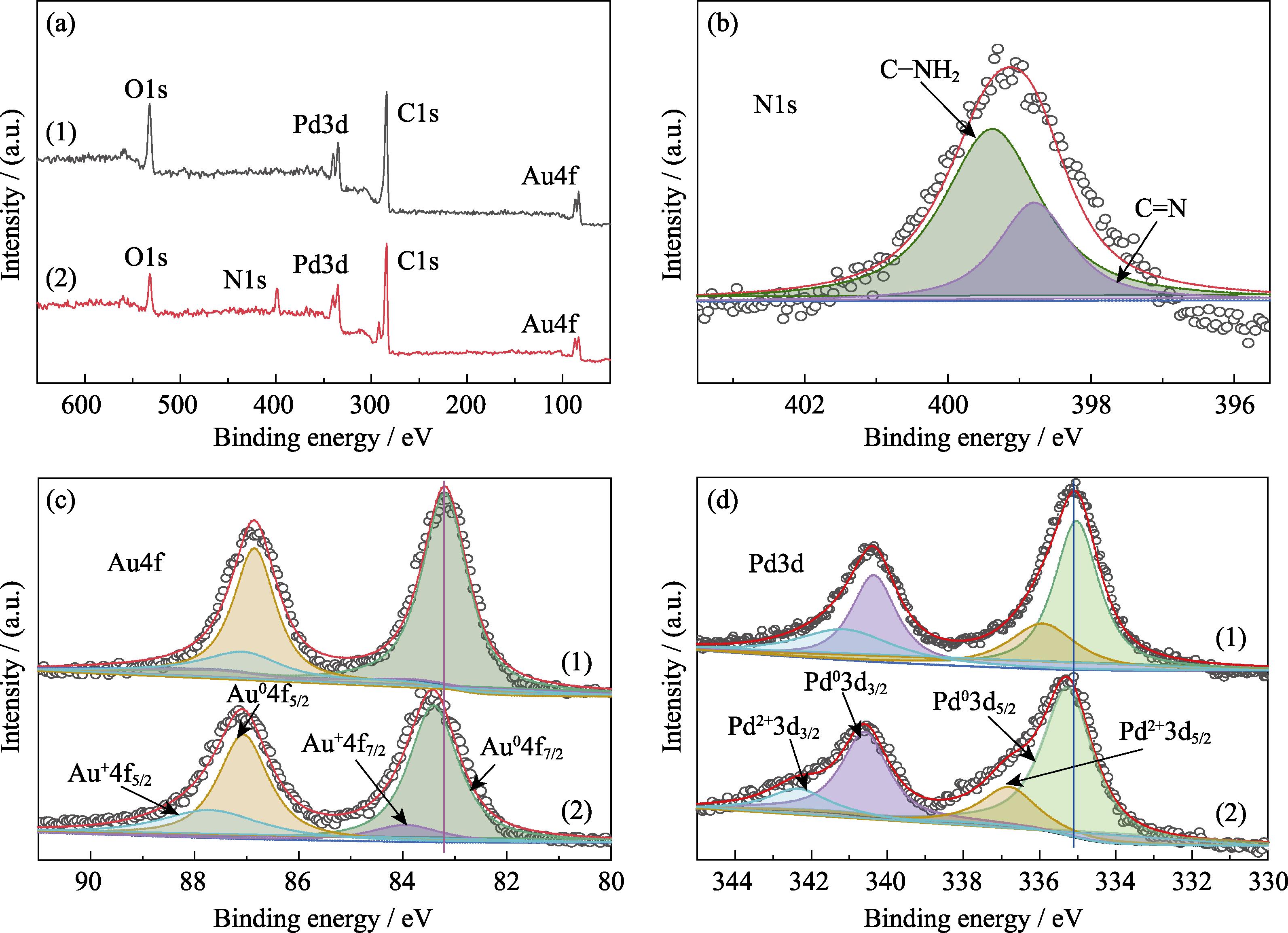
3. XPS spectra of Au0.3Pd0.7/PEI-rGO and Au0.3Pd0.7/rGO
4. Comparison of the catalytic performances for hydrogen evolution from FA dehydrogenation reaction of Au0.3Pd0.7/PEI-rGO, Au0.3Pd0.7/rGO and Au0.3Pd0.7 catalysts
5. (a) Volume of gas versus time for the dehydrogenation of FA at different temperatures over Au0.3Pd0.7/PEI-rGO catalyst; (b) Arrhenius plot (lnTOF versus 1/T ) for Au0.3Pd0.7/PEI-rGO; (c) Volume of gas versus time for the dehydrogenation of FA at different ratios of Aux Pd1-x /PEI-rGO (x =0, 0.1, 0.3, 0.7, 0.9, 1.0) at 323 K; (d) Durability tests of Au0.3Pd0.7/PEI-rGO towards the decomposition of FA (1.0 mol/L, 5.0 mL)
|
Table 1. TOF of different catalysts for FA dehydrogenation

Set citation alerts for the article
Please enter your email address



