Author Affiliations
Renewable Energy Conversion and Storage Center (RECAST), Key Laboratory of Advanced Energy Materials Chemistry (Ministry of Education), College of Chemistry, Nankai University, Tianjin 300071, Chinashow less
1. Influencing factors of X-ray diffraction patterns
2. Schemes of
in situ X-ray diffraction
[20-21] 3. Schematic diagram of the crystal structures of cathode materials
[25⇓⇓-28] 4. Application of XRD Rietveld refinement in cathode materials for lithium-ion batteries
5. Structural evolution during high temperature synthesis of LiNi
0.8Co
0.2O
2 (© 2017, Wiley-VCH)
[41] 6. Temperature-resolved XRD characterization of synthesis process of cathode material LiNi
0.6Co
0.2Mn
0.2O
2 (© 2021, Wiley-VCH)
[43] 7.
In-situ XRD characterization of microwave (MW) hydrothermal synthesis of NCM111(© 2020, AAAS)
[44] 8.
In situ XRD characterization of Ni-rich cathode LiNi
0.8Co
0.1Mn
0.1O
2(NCM811) during charge-discharge (© 2015, ECS)
[49] 9.
In-situ XRD characterization of Li
1.2Ni
0.13Co
0.13Mn
0.54O
2 during charge-discharge process (© 2021, Springer Nature)
[54] 10.
In-situ XRD Rietveld refinement results of LiNi
0.8Co
0.1Mn
0.1O
2 cathode materials during charge and discharge process (© 2022, ECS)
[57] 11. XRD Rietveld refinement results of LiFePO
4 before and after modification (© 2021, RSC)
[63] 12. XRD Rietveld refinement results of Li(Li
0.2Ni
0.2Mn
0.6)O
2 cathode materials (© 2022, Wiley-VCH)
[66] 13. Refined XRD patterns of multiphase materials
| Cathode material | Crystal structure
| Space group
| Cell parameter
| Atom site | Theoretical specific capacity/(mAh·g-1)
| Working voltage/V (vs. Li+/Li)
|
|---|
| LiFePO4 | Olivine | Pnma | a≠b≠c | Li | 4a | 170 | 3.4 | | Fe | 4c | | O | 4c/8d | | LiMn2O4 | Spinel | Fd-3m | a=b=c | Li | 8a | 148 | 4 | | Mn | 16d | | O | 32e | | LiCoO2 | Layer | R-3m | a=b≠c | Li | 3a | 274 | 3.9 | | Co | 3b | | O | 6c | | LiNixCoy(Mn/Al)1-x-yO2 | Layer | R-3m | a=b≠c | Li | 3a | 273-285 | 3.8 | | Ni/Co/Mn/Al | 3b | | O | 6c | | xLi2MnO3·(1-x)LiMO2(0<x<1, M=Ni, Co, Mn) | Layer | R-3m+C2/m | a=b≠c | Li | 2b/2c/4h | 273-350 | 3.8 | | Mn | 4g | | O | 4i/4j |
|
Table 1. Structures and properties of common cathode materials for lithium-ion batteries
[24⇓⇓⇓-28] | Sample | Doped atomic radius/nm
| Displace ion radius/nm
| Lattice constant/nm
| Lattice volume/nm3 | Interatomic distance/nm
| Reliabilityfactor
|
|---|
| LFP | rFe = 0.172 | rFe2+ = 0.074 | a=1.031634b=0.600129c=0.469139 | 0.29045 | Li-O1:0.21664Li-O2:0.20901Li-O3:0.21651Li-O:0.214050 | Rwp = 7.72%Rp =5.63%χ2 = 2.794 | | LFMgP | rMg = 0.172 | rMg2+ = 0.065 | a=1.031583 b=0.600035c=0.469090 | 0.29036 | Li-O1:0.21712Li-O2:0.21041Li-O3:0.21665Li-O:0.214720 | Rwp = 9.32%Rp = 6.79%χ2 = 2.878 | | LFAlP | rAl = 0.182 | rAl3+ = 0.050 | a=1.032204 b=0.600358c=0.469072 | 0.29068 | Li-O1:0.21670Li-O2:0.21001Li-O3:0.21747Li-O:0.214730 | Rwp = 9.14%Rp = 6.60%χ2 = 2.989 | | LFNiP | rNi = 0.162 | rNi2+ = 0.072 | a=1.031083 b=0.599820c=0.468923 | 0.29001 | Li-O1:0.21734Li-O2:0.20863Li-O3:0.21586Li-O:0.213950 | Rwp = 8.26%Rp = 6.12%χ2= 2.929 | | LFVP | rV = 0.192 | rV3+ = 0.074 | a=1.032223 b=0.600494c=0.469485 | 0.291 | Li-O1:0.21864Li-O2:0.21074Li-O3:0.21794Li-O:0.215770 | Rwp = 9.86%Rp = 7.15%χ2= 2.426 |
|
Table 2. Structure refinement result of LiFePO
4 and LiFe
0.95M
0.05PO
4 (M=Mg
2+, Ni
2+, Al
3+, V
3+) (© 2010, EC)
[62] | Sample | a/nm
| b/nm
| c/nm
| V/nm3 |
|---|
| LFP/C | 1.03229 | 0.60061 | 0.46941 | 0.29104 | | LFP/C-YF-1 | 1.03054 | 0.59985 | 0.46903 | 0.28994 | | LFP/C-YF-2 | 1.03082 | 0.59977 | 0.46874 | 0.28980 | | LFP/C-YF-3 | 1.03069 | 0.59989 | 0.46892 | 0.28994 |
|
Table 3. Cell parameters of LiFePO
4 before and after modification by XRD refinement (© 2021, RSC)
[63] | Atom | Site | x | y | z | Occupancy | Uiso |
|---|
| Lia | 3a | 0 | 0 | 0 | 1.000 | 0.014(6) | | Coa | 3b | 0 | 0 | 0.50000 | 1.000 | 0.023(8) | | Oa | 6c | 0 | 0 | 0.2300(6) | 1.000 | 0.049(1) | | Lib | 3a | 0 | 0 | 0 | 0.98(1) | 0.020(1) | | Mgb | 3a | 0 | 0 | 0 | 0.01(9) | 0.020(1) | | Cob | 3b | 0 | 0 | 0.50000 | 0.99(7) | 0.001(2) | | Alb | 3b | 0 | 0 | 0.50000 | 0.002(0) | 0.001(2) | | Tib | 3b | 0 | 0 | 0.50000 | 0.001(0) | 0.001(2) | | Ob | 6c | 0 | 0 | 0.2476(3) | 1.000 | 0.068(5) |
|
Table 4. XRD refinement result of Al, Ti, Mg co-doped LiCoO
2 and bare LiCoO
2 (© 2019, Wiley-VCH)
[64] | Formula | Calculated | Experimental |
|---|
| a/nm
| b/nm
| c/nm
| V/nm3 | a/nm
| V/nm3 |
|---|
| Li8Mn16O32 | 0.886205 | 0.886205 | 0.886205 | 0.695990 | - | - | | Li8Mn15AlO32 | 0.826725 | 0.826725 | 0.826725 | 0.567617 | 0.82507 | 0.561658 | | Li8Mn14Al2O32 | 0.831493 | 0.831493 | 0.799071 | 0.552416 | 0.82466 | 0.560821 | | Li8Mn13Al3O32 | 0.814375 | 0.826337 | 0.820583 | 0.551780 | 0.82110 | 0.553590 |
|
Table 5. XRD structure refinement result of Al doped LiMn
2O
4 (© 2019, Elsevier Ltd.)
[69] 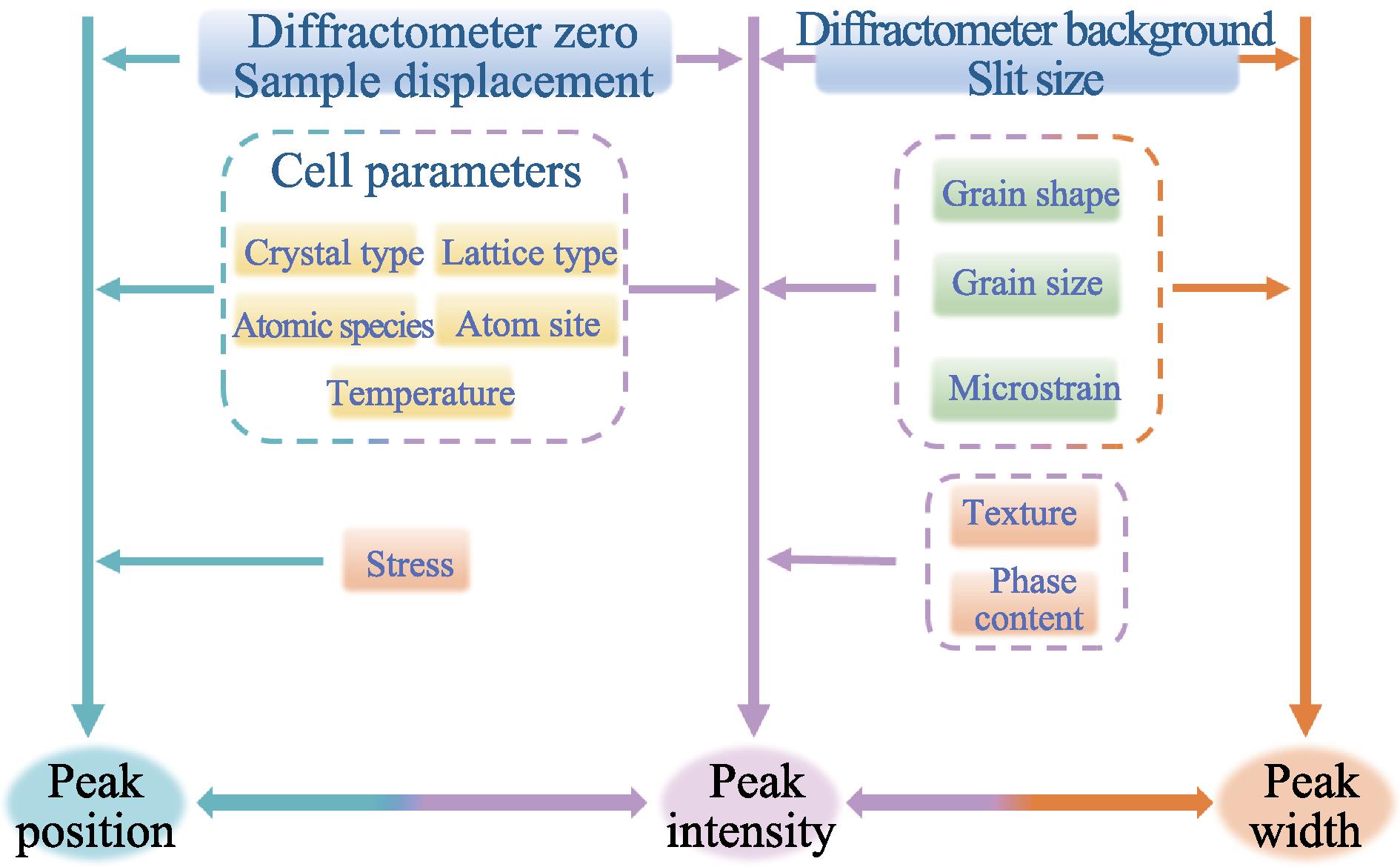
![Schemes of in situ X-ray diffraction[20-21]](/richHtml/jim/2023/38/6/589/img_2.png)




