- Opto-Electronic Advances
- Vol. 6, Issue 11, 230018 (2023)
Abstract
Introduction
Cardiovascular disease is the world's leading cause of death
The distributed optical fiber (DOF) sensing technique represented by the fiber Bragg grating (FBG) is ideally suited for spatiotemporal hemodynamic monitoring. Its spatially distributed multichannel sensing capability and lack of electromagnetic interference lay a foundation for multiple high-SNR physiological signal monitoring
Here, we report a configurable soft microfiber Bragg grating (μFBG) group to monitor spatiotemporal hemodynamics. The precise tapering technology, flexible packaging technology, and advanced femtosecond laser direct-writing technique were employed to prepare the skin-like μFBG patch. Due to the ultrafine diameter and ultrathin thickness, the patch has excellent flexibility and enhanced sensitivity by two orders of magnitude. A ballistocardiogram (BCG) signal is successfully detected by a μFBG patch attached to the chest skin at the tricuspid site. Pulse waves at different superficial artery sites are detected with a high signal-to-noise ratio. The soft μFBG group can be constructed by connecting μFBG patches in series. Benefiting from the flexible layout of the soft μFBG group on the body, systemic PTTs are calculated, revealing the health status of regional arteries. HR and BP, two important hemodynamic parameters, are monitored when the user exercises. Moreover, the variation in regional PR can be detected. Relying on the soft μFBG group, the spatiotemporal hemodynamic monitoring technique opens up new possibilities in daily health management, early screening of disease, and precise treatment in the clinic. Table S1 summarizes the differences of this work from previous studies based on the microfiber sensors.
Results and discussion
Device design and working principle
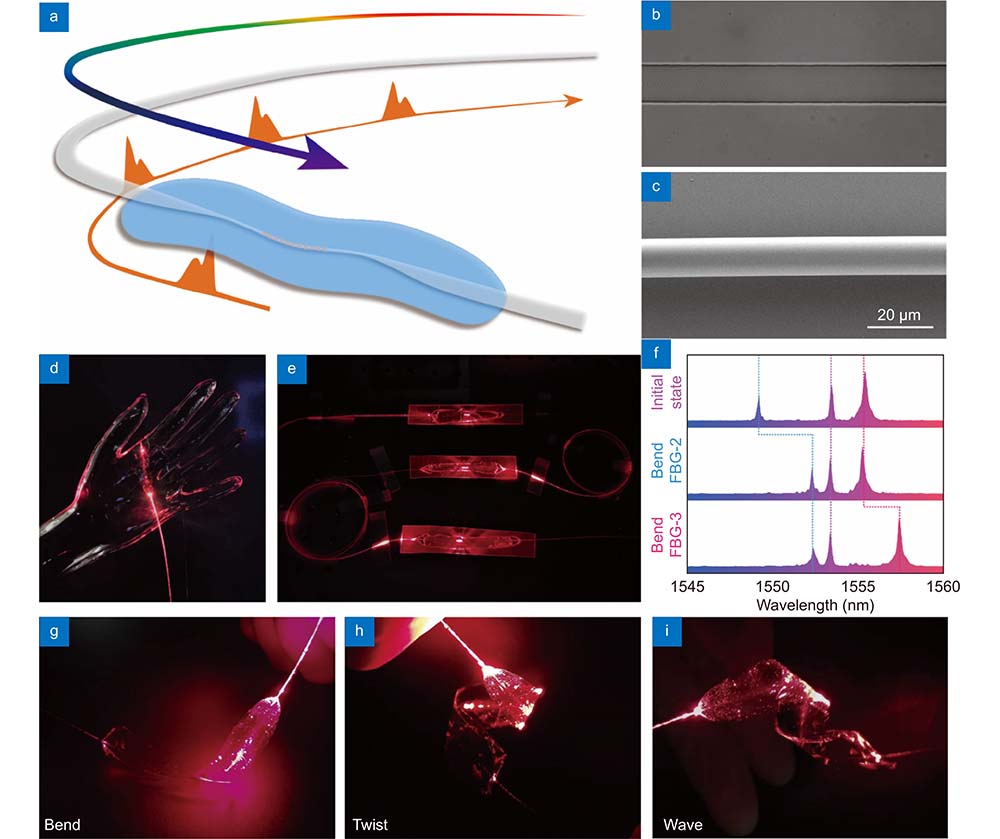
The optical microfiber contains a femtosecond laser-inscribed FBG embedded in a skin-like thin polydimethylsiloxane (PDMS) patch and is connected end-to-end with two commercial single-mode optical fibers (SMFs) for light propagation, as shown in
![]()
Figure 1.
Device characterization
The skin-like μFBG patch converts strain to a shift in the working wavelength, realizing force/vibration sensing. Its response to the stress and vibration was characterized by the stress test equipment (Supplementary information Fig. S2). The soft μFBG patch was attached to a thick Ecoflex (smooth-on 00-30) substrate, which had a low elastic modulus (69 kPa under 100% strain) and was used to simulate human skin. An Ecoflex contactor was employed to compress the soft μFBG patch, resulting in different degrees of deformation.
![]()
Figure 2.
The soft μFBG patch also had a great response to low-frequency vibration, benefiting from the ultrathin thickness of the PDMS patch. As shown in
Human physiological signal detection
Owing to the skin-like mechanical compliance and excellent stress/vibration response, the skin-like μFBG patch can maintain stable contact with human skin in different body locations and detect multiple physiological signals. The subjects were instructed to relax their bodies and to remain calm during the measurement. Ballistocardiogram (BCG) signals, as the near-end signals of the cardiovascular system and pulse waves at multiple far-end body sites, were detected to analyze the propagation process of pulse waves and monitor hemodynamic parameters. An FBG interrogator was employed to record the wavelength shift of the soft μFBG patch caused by micro stress on the skin surface due to cardiovascular activity with a sampling frequency of 2500 Hz.
As shown in
![]()
Figure 3.
When detecting the pulse wave, the soft μFBG patch was attached to the skin at the superficial artery sites, as shown in
The signal amplitude defined by the maximum wavelength shift is a key factor in determining the signal quality. Benefiting from the high sensitivity of the soft μFBG patch, the signal amplitudes of the BCG signal and radial pulse wave were up to 35 pm and 80 pm, respectively, guaranteeing the accuracy of hemodynamic monitoring. Using the same detection method, the commercial FBG had a signal amplitude of less than 2 pm, which was hard to detect by optical signal acquisitions, resulting in poor waveform accuracy (Supplementary information Fig. S5).
Systemic PTT calculation using the μFBG group
Owing to the multichannel and time-synchronized operation capability, the soft μFBG group was built by connecting two μFBG patches in series. The μFBG group was capable of detecting the BCG signal and pulse wave synchronously. The I wave of the BCG signal and the B wave of the pulse wave were used to calculate the PTT. By detecting the pulse wave at different superficial artery sites, systemic PTT was available to thoroughly evaluate the status of the cardiovascular system. As shown in
![]()
Figure 4.
The morphology of pulse waves at the three superficial artery sites differed from each other. The pulse wave at limbs tended to possess a steeper waveform compared with the one at neck. This phenomenon is caused by the backward propagation of pulse waves at arterioles, which is called the amplification effect
The PTTs of the three cases differed from each other, as shown in
Hemodynamic monitoring during exercise
Under the load from exercise, there are many changes in hemodynamics due to increasing metabolism. Research on hemodynamic variation law during exercise can provide an early diagnosis of cardiovascular disease and comprehensively evaluate cardiovascular system function
![]()
Figure 5.
Dynamic peripheral resistance monitoring
PR reflects the unobstructed level of the cardiovascular system, and PR monitoring is significant for the diagnosis of cardiovascular diseases such as arteriosclerosis, thrombus, and hypertension
![]()
Figure 6.
Conclusions
We have demonstrated a soft μFBG group that offers continuous and noninvasive monitoring of spatiotemporal hemodynamics. Combining flexible material integration with precise micro/nanostructure design, the soft μFBG patch achieves both a great sensing response on stress/vibration and intimate coupling with human skin with suitable mechanical compliance. Thanks to its ultrafine diameter and ultrathin thickness, this sensor’s sensitivity has been increased by two orders of magnitude compared to commercial FBGs. Exploiting the advanced femtosecond laser fabrication technique and quasi-distributed optical fiber sensing technique, high-quality all-mechanical physiological signals such as the near-end BCG signal and far-end pulse wave are detected to reflect the actual mechanical process of blood circulation. Relying on the multichannel and time-synchronized operation capability, the soft μFBG group can be used to monitor systemic hemodynamic parameters and reflect regional artery statuses. The device satisfies clinical requirements for real-time and continuous hemodynamic monitoring and reconfigurable regional health evaluation of the cardiovascular system. In addition, electromagnetic immunity makes the μFBG group compatible with essential medical imaging, especially magnetic resonance imaging. Applications in systemic PTT calculation, dynamic hemodynamic monitoring during exercise, and regional PR monitoring demonstrate the great potential in the diagnosis of cardiovascular diseases such as arrhythmia, angiosclerosis, hypertension, and thrombosis and can facilitate precise clinical diagnosis, the fast screening of lesions, and daily health management.
References
[1] GR Dagenais, DP Leong, S Rangarajan, F Lanas, P Lopez-Jaramillo et al. Variations in common diseases, hospital admissions, and deaths in middle-aged adults in 21 countries from five continents (PURE): a prospective cohort study. Lancet, 785-794(2020).
[2] RI Kaur. Electrocardiogram signal analysis - an overview. Int J Comput Appl, 22-25(2013).
[3] SM Debbal, F Bereksi-Reguig. Computerized heart sounds analysis. Comput Biol Med, 263-280(2008).
[4] M Elgendi. On the analysis of fingertip photoplethysmogram signals. Curr Cardiol Rep, 14-25(2012).
[5] J Allen. Photoplethysmography and its application in clinical physiological measurement. Physiol Meas, R1-R39(2007).
[6] D Buxi, JM Redouté, MR Yuce. A survey on signals and systems in ambulatory blood pressure monitoring using pulse transit time. Physiol Meas, R1-R26(2015).
[7] Y Chen, CY Wen, GC Tao, M Bi. Continuous and noninvasive measurement of systolic and diastolic blood pressure by one mathematical model with the same model parameters and two separate pulse wave velocities. Ann Biomed Eng, 871-882(2012).
[8] Y Chen, CY Wen, GC Tao, M Bi, GQ Li. Continuous and noninvasive blood pressure measurement: a novel modeling methodology of the relationship between blood pressure and pulse wave velocity. Ann Biomed Eng, 2222-2233(2009).
[9] XR Ding, BP Yan, YT Zhang, J Liu, N Zhao et al. Pulse transit time based continuous cuffless blood pressure estimation: a new extension and a comprehensive evaluation. Sci Rep, 11554(2017).
[10] R Mukkamala, JO Hahn, OT Inan, LK Mestha, CS Kim et al. Toward ubiquitous blood pressure monitoring via pulse transit time: theory and practice. IEEE Trans Biomed Eng, 1879-1901(2015).
[11] M Sharma, K Barbosa, V Ho, D Griggs, T Ghirmai et al. Cuff-less and continuous blood pressure monitoring: a methodological review. Technologies, 21(2017).
[12] JH Koo, HW Yun, WC Lee, SH Sunwoo, HJ Shim et al. Recent advances in soft electronic materials for intrinsically stretchable optoelectronic systems. Opto-Electron Adv, 210131(2022).
[13] A Bennett, Y Beiderman, S Agdarov, Y Beiderman, R Hendel et al. Monitoring of vital bio-signs by analysis of speckle patterns in a fabric-integrated multimode optical fiber sensor. Opt Express, 20830-20844(2020).
[14] HU Chung, BH Kim, JY Lee, J Lee, ZQ Xie et al. Binodal, wireless epidermal electronic systems with in-sensor analytics for neonatal intensive care. Science, eaau0780(2019).
[15] HU Chung, AY Rwei, A Hourlier-Fargette, S Xu, K Lee et al. Skin-interfaced biosensors for advanced wireless physiological monitoring in neonatal and pediatric intensive-care units. Nat Med, 418-429(2020).
[16] Y Jin, GN Chen, KT Lao, SH Li, Y Lu et al. Identifying human body states by using a flexible integrated sensor. npj Flex Electron, 28(2020).
[17] HC Li, YJ Ma, ZW Liang, ZH Wang, Y Cao et al. Wearable skin-like optoelectronic systems with suppression of motion artifacts for cuff-less continuous blood pressure monitor. Natl Sci Rev, 849-862(2020).
[18] F Zhong, W Hu, PN Zhu, H Wang, C Ma et al. Piezoresistive design for electronic skin: from fundamental to emerging applications. Opto-Electron Adv, 210029(2022).
[19] JH Li, JH Chen, F Xu. Sensitive and wearable optical microfiber sensor for human health monitoring. Adv Mater Technol, 1800296(2018).
[20] CH Wang, XS Li, HJ Hu, L Zhang, ZL Huang et al. Monitoring of the central blood pressure waveform via a conformal ultrasonic device. Nat Biomed Eng, 687-695(2018).
[21] L Zhang, J Pan, Z Zhang, H Wu, N Yao et al. Ultrasensitive skin-like wearable optical sensors based on glass micro/nanofibers. Opto-Electron Adv, 190022(2020).
[22] HT Zhu, LW Zhan, Q Dai, B Xu, Y Chen et al. Self‐assembled wavy optical microfiber for stretchable wearable sensor. Adv Opt Mater, 2002206(2021).
[23] KO Hill, G Meltz. Fiber Bragg grating technology fundamentals and overview. J Lightwave Technol, 1263-1276(1997).
[24] Ł Dziuda, FW Skibniewski. A new approach to ballistocardiographic measurements using fibre Bragg grating-based sensors. Biocybern Biomed Eng, 101-116(2014).
[25] Y Haseda, J Bonefacino, HY Tam, S Chino, S Koyama et al. Measurement of pulse wave signals and blood pressure by a plastic optical fiber FBG sensor. Sensors, 5088(2019).
[26] L Xu, N Liu, J Ge, XQ Wang, MP Fok. Stretchable fiber-Bragg-grating-based sensor. Opt Lett, 2503-2506(2018).
[27] EA Al-Fakih, Osman NA Abu, Adikan FR Mahamd, A Eshraghi, P Jahanshahi. Development and validation of fiber Bragg grating sensing pad for interface pressure measurements within prosthetic sockets. IEEE Sensors J, 965-974(2016).
[28] TL Li, YF Su, FY Chen, XQ Liao, Q Wu et al. A skin‐like and highly stretchable optical fiber sensor with the hybrid coding of wavelength–light intensity. Adv Intell Syst, 2100193(2022).
[29] J Pan, Z Zhang, CP Jiang, L Zhang, LM Tong. A multifunctional skin-like wearable optical sensor based on an optical micro-/nanofibre. Nanoscale, 17538-17544(2020).
[30] SQ Ma, XY Wang, P Li, N Yao, JL Xiao et al. Optical Micro/Nano fibers enabled smart textiles for human–machine interface. Adv Fiber Mater, 1108-1117(2022).
[31] G Brambilla, V Finazzi, DJ Richardson. Ultra-low-loss optical fiber nanotapers. Opt Express, 2258-2263(2004).
[32] JY Lou, YP Wang, LM Tong. Microfiber optical sensors: a review. Sensors, 5823-5844(2014).
[33] J Thomas, C Voigtländer, RG Becker, D Richter, A Tünnermann et al. Femtosecond pulse written fiber gratings: a new avenue to integrated fiber technology. Laser Photonics Rev, 709-723(2012).
[34] JX Luo, S Liu, PJ Chen, SZ Lu, Q Zhang et al. Fiber optic hydrogen sensor based on a Fabry-Perot interferometer with a fiber Bragg grating and a nanofilm. Lab Chip, 1752-1758(2021).
[35] G Brambilla, F Xu, P Horak, Y Jung, F Koizumi et al. Optical fiber nanowires and microwires: fabrication and applications. Adv Opt Photonics, 107-161(2009).
[36] LM Tong, F Zi, X Guo, JY Lou. Optical microfibers and nanofibers: a tutorial. Opt Commun, 4641-4647(2012).
[37] LY Yang, YP Li, F Fang, LY Li, ZJ Yan et al. Highly sensitive and miniature microfiber-based ultrasound sensor for photoacoustic tomography. Opto-Electron Adv, 200076(2022).
[38] R Mukkamala, D Xu. Continuous and less invasive central hemodynamic monitoring by blood pressure waveform analysis. Am J Physiol Heart Circ Physiol, H584-H599(2010).
[39] S Laurent, P Boutouyrie, R Asmar, I Gautier, B Laloux et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension, 1236-1241(2001).
[40] J Kim, TJ Song, D Song, KJ Lee, EH Kim et al. Brachial-ankle pulse wave velocity is a strong predictor for mortality in patients with acute stroke. Hypertension, 240-246(2014).
[41] GQ Zhang, MW Gao, D Xu, NB Olivier, R Mukkamala. Pulse arrival time is not an adequate surrogate for pulse transit time as a marker of blood pressure. J Appl Physiol, 1681-1686(2011).
[42] PA Rueckert, PR Slane, DL Lillis, P Hanson. Hemodynamic patterns and duration of post-dynamic exercise hypotension in hypertensive humans. Med Sci Sports Exerc, 24-32(1996).
[43] Å Ohlsson, D Steinhaus, B Kjellström, L Ryden, T Bennett. Central hemodynamic responses during serial exercise tests in heart failure patients using implantable hemodynamic monitors. Eur J Heart Fail, 253-259(2003).
[44] HD Intengan, EL Schiffrin. Structure and mechanical properties of resistance arteries in hypertension: role of adhesion molecules and extracellular matrix determinants. Hypertension, 312-318(2000).
[45] AJ Taylor, A Bobik, MC Berndt, D Ramsay, G Jennings. Experimental rupture of atherosclerotic lesions increases distal vascular resistance: a limiting factor to the success of infarct angioplasty. Arterioscler Thromb Vasc Biol, 153-160(2002).
Set citation alerts for the article
Please enter your email address



