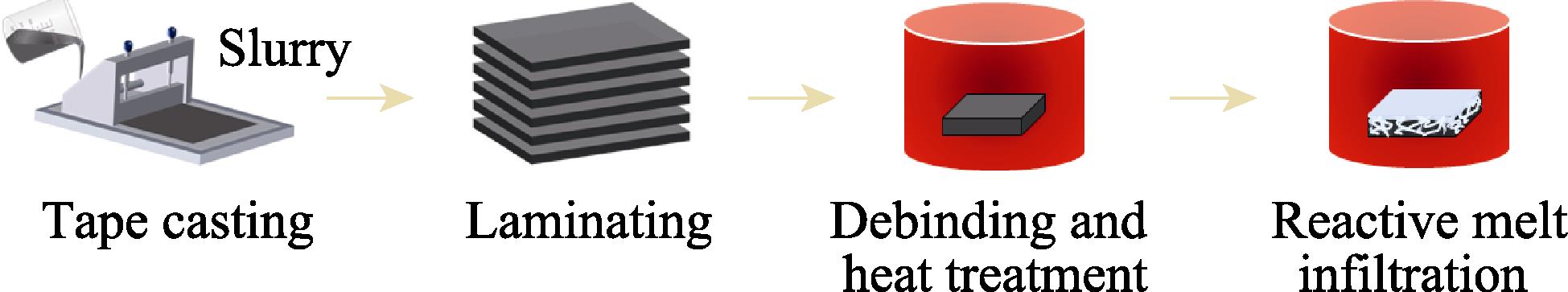
Min TAN, Xiaowu CHEN, Jinshan YANG, Xiangyu ZHANG, Yanmei KAN, Haijun ZHOU, Yudong XUE, Shaoming DONG. Microstructure and Oxidation Behavior of ZrB2-SiC Ceramics Fabricated by Tape Casting and Reactive Melt Infiltration [J]. Journal of Inorganic Materials, 2024, 39(8): 955
Search by keywords or author
- Journal of Inorganic Materials
- Vol. 39, Issue 8, 955 (2024)
Abstract
View in Article
View in Article
View in Article
View in Article
View in Article
Set citation alerts for the article
Please enter your email address



